Conventional nitriding of low-density powder metal parts using salt or ammonia is difficult because salt or sand may be trapped in the porous structure and must be removed. Also, ammonia penetrates the internal structure of a powder metal part and causes thru hardening, embrittlement, and swelling.
Nitride Compound Zone Improves Powder Metals' Wear & Corrosion Resistance
The most important portion of the nitrided layer in a low-alloy powder metal part is the compound zone formed by the Fe4N and Fe2-3N iron nitrides and Fe2-3N(C) nitrocarbides. This zone has very good frictional and anti-corrosion properties and can easily be seen under the optical microscope in Figure 1.
Ion Nitriding Works Well for High Porosity Powder Metal
Presence of a high porosity level and passive surface layer makes stainless steel powder metal products very good candidates for UltraGlow® Ion Nitriding. Products made of powder metal stainless steel do not form a thick compound zone or a very deep diffusion zone, but they have a much higher hardness after UltraGlow® Ion Nitriding than carbon-copper-iron alloys.
Pores in Powder Metal Do Not Affect Nitride's Ability to Form Consistent, Uniform Nitride Layer
The microstructure of the nitrided layer in stainless steel contains very small nitrides and clusters, which causes the steel to etch darker. Pores present in the powder metal steel do not affect its ability to form a consistent, uniform nitride layer, and even alloys containing a very extensive amount of alloying elements can be UltraGlow® Ion Nitrided as shown in Figure 2.
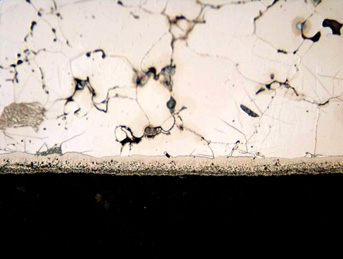
Figure 1: Photomicrograph of the UltraGlow® Ion Nitrided Fe-0.8% Parmature. Note porous nature of the alloy. Etched with 2% nital.
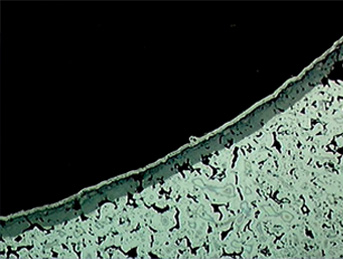
Figure 2: Photomicrograph of the narrow slot structure in the AISI type 310 stainless steel PM unison ring after UltraGlow® Ion Nitriding at 1080° F (580° C). Note the porous nature of the alloy. The white layer on the surface is a nickel coating applied for better edge retention during metallographic sample preparation. Magnification 160X. Etched with 3% nital.
Learn more about Nitriding Powder Metal by clicking the button below.
VIEW RESOURCES
Nitriding Powder Metal References
- Figure 1 above was taken from E. Rolinski, A. Koniczny, G. Sharp, “Nature of Surface Changes in Stamping Tools of Gray and Ductile Cast Iron During Gas and Plasma Nitrocarburizing”, Journal of Materials Engineering and Performance, Vol. 18(8) Nov. 2009, p. 1052-1059.
- Figure 2 above was taken from E. Rolinski, G. Sharp, “Ion Nitriding and Nitrocarburizing of Sintered PM Materials”, Industrial Heating, October 2004, p. 33-35.