posted
On Friday, August 16, 2024
in
Blog
 It is estimated that corrosion costs 2.5 trillion dollars a year globally1. On a smaller scale, corrosion is a large financial and time drain due to replacement and repair of damaged parts. Thus, there is a constant need to improve the corrosion resistance of metals.
It is estimated that corrosion costs 2.5 trillion dollars a year globally1. On a smaller scale, corrosion is a large financial and time drain due to replacement and repair of damaged parts. Thus, there is a constant need to improve the corrosion resistance of metals.
Advanced Heat Treat Corp. (AHT) offers several heat treatment processes to improve corrosion resistance of customer parts; however, each process’ effects vary depending on the material, requiring AHT to research and test these differences.
For many years, AHT has used the industry standard Salt Spray (Fog) corrosion testing to research the heat treatments’ effects on the corrosion properties of various carbon and stainless steels. Despite being the industry standard, the salt spray test has several limitations in data collection as shown in the section below2. To overcome these limitations and further research the heat treatments’ effects on corrosion properties, AHT has implemented the use of a potentiostat.
Combining the two types of tests – potentiostat and salt spray - yields a more complete analysis of corrosion failures.
What is a Potentiostat?
A potentiostat is a tool used in Electrochemical Impendence Spectroscopy (EIS) to measure the corrosive behavior of metals. A potentiostat testing cell is shown above. It consists of two electrodes, a beaker filled with a NaCl solution, and the exposed metal surface of interest.
The potentiostat applies a voltage across the system, sweeps it through a specified range, and measures the current through the cell at each voltage. The cell current is proportional to the rate of the corrosion redox reactions, from which several corrosion properties of the material can be determined.
Potentiostat vs. Salt Spray Corrosion Testing2
|
Potentiostat
(Quantitative Data)
|
Salt Spray
(Qualitative Data)
|
Standard Used
|
ASTM G61
|
ASTM B117
|
Run Time
|
2 - 3 Hours
|
500+ Hours
|
Data Collected
|
-
Pitting Potentials
-
Corrosion Rate
-
Failure Type
|
-
Pitting Occurrence
-
Time to Failure
|
Potentiostat Data
Shown below is an example of a potentiodynamic scan of two 4140 samples, presented as a Tafel plot in which the applied voltage is plotted against the measured absolute current. Graphed in green is the untreated sample and the blue is processed with UltraOx treatment.
There are two key features in the graph, each indicated by the colored arrows. The first feature is the open circuit potential, as indicated by the black arrows. This is referred to as the nobility of a sample surface, and it represents the electric potential (voltage) at which the current passes through zero due to reaching equilibrium between the oxidation and reduction reactions. A higher (more positive) value indicates a higher resistance to oxidation or surface corrosion2.
The second feature is the pitting potential, as indicated by the blue arrow. The pitting potential is defined as the voltage at which there is a sudden increase in current. Similar to the open circuit potential, a higher pitting potential indicates a higher resistance to pitting corrosion2. Data containing multiple pitting potentials is indicative of a heterogenous or multi-layer sample, as each potential corresponds to a unique layer experiencing pitting.
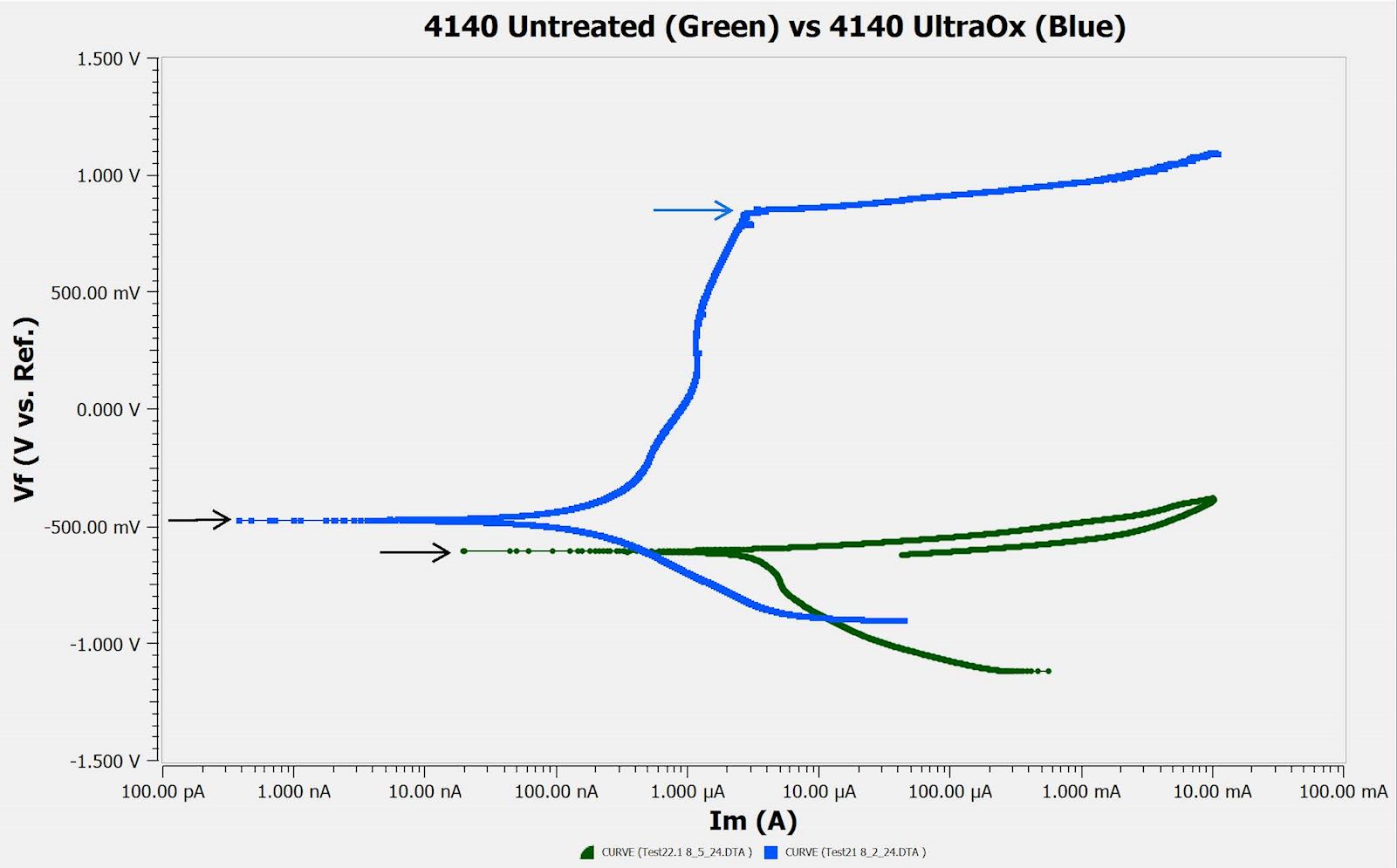
UltraOx Improves Corrosion & Pitting Resistance of 4140 Alloy Steel
In this particular example, the UltraOx data has a higher open circuit potential meaning a superior surface. Additionally, the untreated sample shows no resistance to pitting, while the UltraOx sample only begins to show pitting at over 800 mV. Thus, the data suggests that the UltraOx heat treat process provides greater resistance to oxidation and pitting.
Questions about Salt Spray Corrosion Testing, the Potentiostat or UltraOx?
CONTACT US
References
[1] Koch, G. (2017, January 1). 1 - Cost of corrosion (A. M. El-Sherik, Ed.). ScienceDirect; Woodhead Publishing. https://www.sciencedirect.com/science/article/abs/pii/B9780081011058000012
[2] R. Johnson, Introduction to Corrosion and Electrochemical Testing, Aug 8, 2019
- corrosion resistance
- ultraox®